Introduction:Strong tonic signaling caused by spontaneous CAR aggregation is an important mechanism of CAR-T cell exhaustion which can impair the anti-tumor function. Many studies have illustrated the features of CAR-T cell activated excessively by tonic signaling at transcriptional, protein and epigenetic levels. Regulation of tonic signaling and downstream responses could enhance the anti-tumor effect. However, post-transcriptional features is little known while a lot of studies have demonstrated that post-transcripitonal processes such as exon skipping (ES), intron removal and alternative polyadenylation (APA) play critical roles in T cell activation and homeostasis.
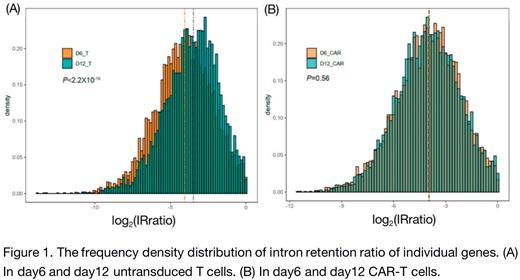
Methods and results:To investigate post-transcriptional features of CAR-T cell excessively activated by tonic signaling, we performed RNA sequencing on CAR-T cells and untransduced T cells at day6 or day12 after anti-CD3/CD28 beads stimulation. These samples were sequenced with high read depth (>80 M reads) to enable accurate quantification of alternative splicing in downstream analysis. Exon skipping events were identified by rMATS (≥10 splice junction reads per event, |dPSI|>0.1 and p value <0.05), then we predicted whether these events affect protein domains and protein-protein interactions. We performed enrichment analysis based on these affected interaction edges. From early to late stage after stimulation, CAR-T cells underwent exon skipping events which were significantly enriched in pathways such as cytokine signaling, calcium mobilization and adaptive immune system. Conversely, untransduced T cells from early to late stage also underwent amounts of ES events while the affected interactions were rarely enriched in meaningful pathways. Previous study has discovered that T cell activation could induce global reduction of intron retention (IR) level and intron-retained transcripts are less stable than fully spliced transcripts (Ni Ting et al. Nucleic acids research 2016). We found that IR level was in line with the activation level of the these groups. As CAR-T cells were continuously activated from day6 to day12, the global IR level didn't changed significantly while untransduced T cells has significantly elevated the global IR level (Fig.1). We also investigated the features of alternative polyadenylation which occurred in over 70% of mammalian mRNA genes, turning out expression of alternative polyadenylation isoforms with different 3′UTR lengths. A total of 448 genes showed a significant difference (P-value <0.05, a relative abundance change >5%)in the proximal-to-distal poly(A) site use between day6 and day12 CAR-T cells. Compared to day6, 229 genes increased use of the proximal poly(A) site and 219 genes increased use of the distal poly(A) site in day12 CAR-T cells. In untransduced T cells, we found that the number of genes showing lengthening 3′UTRs was 4.1-fold greater than the number of genes showing the opposite trend (589 vs. 143), indicating the general 3′UTR lengthening in the cell. Previous studies have described that T cell activation can cause global 3′UTR shortening and suggested that longer 3′UTR may expose more miRNA binding sites which impaired the stability of RNA (Sandberg R et. al. Science 2009). As all above post-transcriptional features we found seemed to contribute to activation signal and RNA stability, we asked whether we could prevent excessive activation and exhaustion by regulating post-transcriptional process. We treated CAR-T cells with madrasin for 72 hours which is a splicing inhibitor interfering with the early stages of spliceosome assembly. We found that madrasin at a low concentration can significantly inhibit the CAR-T cells' activation, exhaustion, terminal differentiation without affecting the apoptosis rate.
Significance: We have discovered the importance of efficient post-transcriptional machinery during the process of CAR-T cell being excessively activated by tonic signaling.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal